Abstract
Background
The role of allogeneic stem cell transplantation in the management of myeloma remains uncertain as it is a procedure with significant mortality and low long term rates of disease control. We sought to evaluate the feasibility of using adjunctive Lenalidomide (Revlimid®) to augment long term myeloma response following Reduced-Intensity Conditioned (RIC) allograft in the UK NCRI trial LenaRIC (Lenalidomide in Reduced Intensity Conditioned Transplant)-ISRCTN:16228367.
Material & Methods
Forty (40) patients were recruited in this single arm phase II trial at ten UK stem cell transplant centres from June 2011-June 2015. All patients were in CR/VGPR 1 or 2 and had undergone a Melphalan conditioned autologous transplant in the previous 6 months. They then underwent a RIC allograft using Fludarabine & 2Gy TBI with ATG (Fresenius) as in vivo T-cell depletion (planned tandem SCT programme). Patients were scheduled to receive Lenalidomide (10mg until 2013, reduced to 5mg thereafter on DMEC advice) from day +35 if stable donor engraftment was present in the absence of GvHD. Lenalidomide was planned to be discontinued 12 months post-transplant with donor lymphocyte infusions given to patients with evidence of residual disease, relapse or mixed chimerism.
Results
Of the thirty nine(39) patients transplanted within 42 days of study entry, 8 were in CR1, 16 in VGPR1, 7 in CR2 and 8 in VGPR2. The median age was 49 years (range 35-65) with peripheral blood as the graft source in the 39 patients, from either sibling (n=16) or 10/10 matched unrelated donor (n=23). 1 patient failed to proceed to transplant. Of thirty four (34) evaluable patients, 28 (82.4%) achieved full donor T-cell chimerism and 32 (94.1%) full donor in whole blood. 34 patients completed at least one cycle of Lenalidomide. Mean number of cycles received was 7.4 (range 1-12, median 9.0). Mean dose was 5.2mg, median 5mg. 16 patients completed at least 10 cycles and 5 patients completed 12 cycles as per protocol treatment; of those completing >= 10 cycles, 5/16 received DLI.
Safety data
There were eight (8) patients who had biopsy-proven aGVHD (4 of grade 1-2 and 4 of grade 3- all 4 within 3 cycles of Lenalidomide) and 3 patients with cGVHD (2 extensive and 1 limited). No Grade 3/4 aGVHD was seen following DMEC advice to reduce the Lenalidomide dose to 5mg daily. 8 patients experienced grade 3 or 4 non-Haem toxicity. The majority of AEs were grade 1-2. Significant (Grade 3/4) non-Haem SAEs in this transplant population included Gastro-intestinal (4 patients), metabolism (4 patients), infection (13 patients) and vascular (4 patients). One year relapse-free survival was 84.3%.
Clinical Outcomes
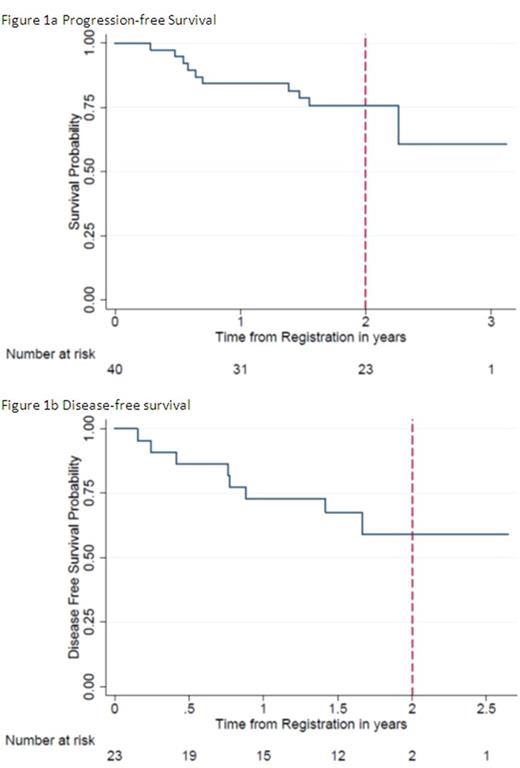
Efficacy was assessed at a median follow-up of 445 days. The pre-transplant CR rate of 38.4% was improved to 67.6% at best response post-transplant. PFS at 2 years was 43.1% (figure 1a), OS at years 75.7% with a 2 year disease-free survival of 59.1% (figure 1b). Of the 10 patients who have died, 6 have been recorded as disease-related.
Conclusions
1) Based on the pre-defined safety stopping rules, the LenaRIC study shows that post-graft Lenalidomide is safe and tolerated in a T-depleted RIC setting at a dose of 5mg daily- this results in a tolerable AE profile with an acceptable GVHD rate.
2) Acute GVHD only occurred in the first 3 cycles of exposure to Lenalidomide- consideration should be given to examining the longer term use of Lenalidomide beyond 12 months following a T-depleted RIC allograft to augment long term outcomes
3) CR rates were nearly doubled by this approach
4) Further work correlating Lenalidomide exposure with clinical events and immune reconstitution is ongoing.
An unrestricted educational grant was provided to support the trial & adjunctive science by Cancer Research UK and by Celgene. Lenalidomide provided free of charge by Celgene. Lenograstim provided free of charge by Chugai. The support and time of participating patients and their families is gratefully acknowledged.
Cook: Janssen: Honoraria, Other: Travel support, Research Funding; Amgen: Honoraria, Other: Travel support; Takeda: Honoraria; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Other: Travel support, Research Funding; Jazz Pharmaceuticals: Honoraria. Brock: Merck: Other: travel expenses; Roche: Honoraria, Other: travel expenses; GlaxoSmithKline: Equity Ownership; Astra-Zeneca: Equity Ownership. Snowden: Sanofi: Honoraria. Hunter: Celgene: Other: Travel Support. Cavenagh: Takeda: Consultancy; Novartis: Consultancy; Janssen: Honoraria; Celgene: Consultancy. Cook: BMS: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal